Introduction :
In
1819, Pierre-Louis Dulong and Alexis-Therese Petit, of France first
defined specific heat.The heat capacity per unit mass of a body is
called Specific heat.
Specific Heat Definition :
Specific heat is the quantity of heat required to alter the temperature by one degree of a unit mass of a substance.Specific heat capacity c, the heat capacity per unit mass, C= J/Kg. K
Specific Heat Definition :
Specific heat is the quantity of heat required to alter the temperature by one degree of a unit mass of a substance.Specific heat capacity c, the heat capacity per unit mass, C= J/Kg. K
Specific Heat of Water
Specific heat of Water is high when compared to other liquids; and this helps water to stabilize temperatures.The specific heat of water = 4.184 Joules per gram per degree centigrade.
Image on Specific heat of liquid water
When
water loses or absorbs a given amount of heat, there is a very slight
change in its temperature because of the high specific heat of water as
compared to other materials. When water is boiled in an iron pot,
while the iron pot feels hot, the water in it is still lukewarm since
the specific heat of water when compared to iron is ten times higher.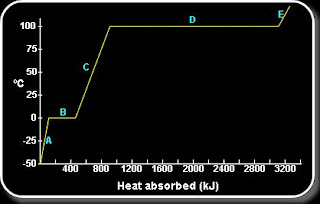
No comments:
Post a Comment